Nanotherapeutic Shows Promise in Eliminating Lethal Tumors
November 27, 2013

Although these "spherical nucleic acids" are not magnetic, maybe we can do the same thing with very small magnetic nanoparticles. Try!
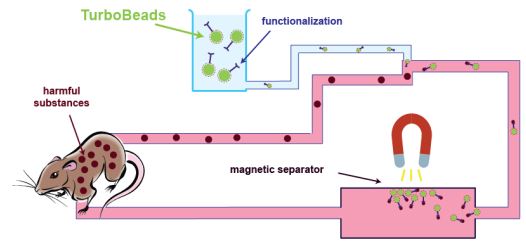
Nanomagnet-Based Removal of Digoxin and Lead from Living Rats
November 22, 2013Inge Herrmann et al. reported the in vivo (rat) purification of blood from Pb and digoxin. For this TurboBeads were functionalized with an iminodiacetic acid-based chelator respectively a digoxin antibody FAB (digiFAB). In detail, the nanoparticles were injected into an extracorporeal purification process. The functionalized magnetic beads bound the harmful substances and the actual seperation took place with the help of an applied magnetic field. A 50 % drop of concentration of both lead and digoxin could be achieved as fast as 10 minutes.
Get the details here: Nanoscale 2013, 5, 8718. (DOI)
The same group also reported the in vitro purification of blood from endotoxins via polymyxin B-functioanlized highly magnetic TurboBeads. The nanoparticles were added to endotoxin spiked blood and were thereafter magnetically separated from the solution. The efficient removal of endotoxins from blood by this straight forward procedure was investigated by ELISA.
Special Issue about Magnetic Nanoparticle Hyperthermia
November 13, 2013
 In 1957 an American surgeon, Robert Gilchrist ignited excitement by suggesting the possibility that cancer can be treated with magnetic nanoparticle hyperthermia. Magnetic nanoparticles provide unique capabilities for biomedical research and clinical applications, some of which have been realized within the past two or three years. Much greater potential exists, but challenges must be addressed.
In 1957 an American surgeon, Robert Gilchrist ignited excitement by suggesting the possibility that cancer can be treated with magnetic nanoparticle hyperthermia. Magnetic nanoparticles provide unique capabilities for biomedical research and clinical applications, some of which have been realized within the past two or three years. Much greater potential exists, but challenges must be addressed.
A just published special issue of the International Journal of Hyperthermia brings together original research and review articles from leaders in the field whose contributions highlight recent progress and future directions for cancer research and therapy with magnetic iron oxide nanoparticles. Dr. Robert Ivkov did a great job at editing this issue (Volume 29, Number 8, December 2013).
Check out all the different articles here:
http://informahealthcare.com.ezproxy.library.ubc.ca/toc/hth/29/8
Catalytic Nickel and Cobalt Nanowires
November 04, 2013
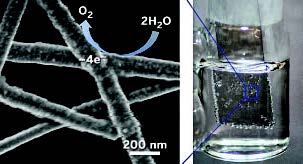 If we could convert sun power to hydrogen in an inexpensive and sustainable way, we would have an environmentally friendly fuel - forever! Steps are now taken towards this goal at Duke University by chemists Zuofeng Chen, Benjamin J. Wiley , and coworkers have. In one potentially low-cost method, sunlight enters a specially designed cell and triggers a multistep electrochemical process that begins with water oxidation on a catalyst surface. That reaction produces O2 and frees electrons and protons, which can be combined to yield H2 .
If we could convert sun power to hydrogen in an inexpensive and sustainable way, we would have an environmentally friendly fuel - forever! Steps are now taken towards this goal at Duke University by chemists Zuofeng Chen, Benjamin J. Wiley , and coworkers have. In one potentially low-cost method, sunlight enters a specially designed cell and triggers a multistep electrochemical process that begins with water oxidation on a catalyst surface. That reaction produces O2 and frees electrons and protons, which can be combined to yield H2 .
These photoelectrochemical cells often depend on indium tin oxide (ITO), a transparent, electrically conductive material, coated with a catalytic metal oxide. Because of its ability to serve as a transparent electrode, ITO is the go-to material for various photoelectrochemical processes. But indium and ITO are expensive. In addition, the catalytic oxide layer reduces ITO’s light transmission and thus cell efficiency, and its conductivity falls with extended use. The Duke team developed copper nanowires coated with a shell of nickel or cobalt as successful replacement. Initial tests show that a mesh of this inexpensive, transparent, conductive material exhibits sustained water oxidation activity comparable to that of metal oxide films, but transmits nearly seven times more light. In addition, the material can be deposited on glass or plastic substrates via simple liquid-phase methods, which may lead to new types of robust, flexible photoelectrochemical cells and other devices. Check out the original paper here.
Magnetic Responsive Polymer Composite Materials
October 23, 2013
Magnetic responsive materials are the topic of intense research due to their potential breakthrough applications in the biomedical, coatings, microfluidics and microelectronics fields. By merging magnetic and polymer materials one can obtain composites with exceptional magnetic responsive features. Magnetic actuation provides unique capabilities as it can be spatially and temporally controlled, and can additionally be operated externally to the system, providing a non-invasive approach to remote control.
The authors identified three classes of magnetic responsive composite materials, according to their activation mode and intended applications, which can be defined by the following aspects. (A) Their ability to be deformed (stretching, bending, rotation) upon exposure to a magnetic field. (B) The possibility of remotely dragging them to a targeted area, called magnetic guidance, which is particularly interesting for biomedical applications, including cell and biomolecule guidance and separation. (C) The opportunity to use magnetic induction for thermoresponsive polymer materials actuation, which has shown promising results for controlled drug release and shape memory devices. For each category, essential design parameters that allow fine-tuning of the properties of these magnetic responsive composites are presented using key examples.
Otomagnetics Receives Inaugural Rybski MedTech Award
October 05, 2013
.jpg) Washington, DC - On October 1, 2013, the Advanced Medical Technology Association (AdvaMed) announced that Otomagnetics – a start-up firm that has developed a “magnetic syringe” that directs therapy to hard-to-reach locations – has been awarded the inaugural Virginia Shimer Rybski Memorial Award, recognizing the potential of a promising entrepreneur or entrepreneurial company. This award is worth $10,000.
Washington, DC - On October 1, 2013, the Advanced Medical Technology Association (AdvaMed) announced that Otomagnetics – a start-up firm that has developed a “magnetic syringe” that directs therapy to hard-to-reach locations – has been awarded the inaugural Virginia Shimer Rybski Memorial Award, recognizing the potential of a promising entrepreneur or entrepreneurial company. This award is worth $10,000.
Otomagnetics, a University of Maryland-College Park start-up, is commercializing a platform technology to minimally-invasively direct therapeutic payloads to hard-to-reach anatomical targets. The first target for the company’s proprietary magnetic delivery system is the middle and inner ear, to treat conditions such as sudden hearing loss, tinnitus and middle ear infections. Other potential markets include drug delivery to the eye, the dental market, and neuro-degenerative diseases.
Otomagnetics was chosen to receive the first-ever Virginia Shimer Rybski Memorial Award since “the company focused on a specific clinical issue with a significant unmet need,” said award judge Dr. Jeffrey Hausfeld, chairman and founder of the Society of Physician Entrepreneurs. “We appreciate the science of non-invasively directing bio-compatible nano-particles and payloads through tissue in order to bring therapeutics to hard-to-reach targets such as the inner ear. The judges appreciated the fact that this is a novel, platform technology and that Dr. Shapiro did an excellent job at conveying the promise of a very useful clinical device that will hopefully improve patient care and outcomes.”
The technology was developed by a team of researchers led by Dr. Benjamin Shapiro at the Fischell Department of Bioengineering and the Institute for Systems Research, at the University of Maryland at College Park. Financial support was provided by the State of Maryland including the Technology Development Corporation and Maryland Industrial Partnerships, as well as from the UK-based Action on Hearing Loss and the Sheikh Zayed Institute for Pediatric Surgical Innovation at Children’s National Health System in Washington, DC.
“Our goal is to move our magnetic delivery technology from the lab to the market so that it can help patients,” said Dr. Shapiro. “To have a panel of experts, from clinicians to investors and regulatory advisors, see the potential of our approach and award us the first-ever Virginia Shimer Rybski prize, that is a distinction and an honor for us. It will certainly aid our efforts to develop this technology.”
Stem Cell Extraction Technique Uses Magnets
October 03, 2013Neural stem cells have been magnetically extracted from a rat's brain without harming the rodent, offering hope that the procedure can be used to harvest stem cells from humans. Edman Tsang of the University of Oxford developed a technique in which magnetic nanoparticles coated with antibodies that cling to neural stem cells are injected into the lateral ventricles of the rats' brains. After the nanoparticles attach themselves to the stem cells, a magnetic field around the rats' heads pulls the cells together so they can be extracted with a syringe.
For more details: Check here.
Smartphone Takes Fluorescent Nanoparticle Pictures
October 02, 2013
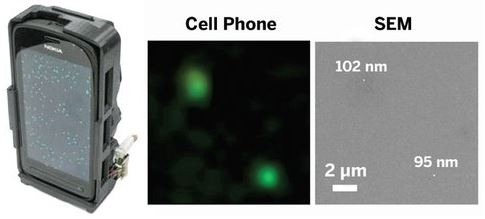
The device, designed by Aydogan Ozcan of UCLA and colleagues, consists of a laser diode to excite fluorescent dyes and a compact system of lenses and filters that remove background noise created by the laser light. The team used the microscope to detect human cytomegalovirus particles, which are between 150 and 300 nm in diameter, and polystyrene beads, which at about 100 nm in diameter were the smallest objects that could be detected. According to Ozcan, the mini microscope is the first portable, cell-phone-based imaging system sensitive enough to resolve individual nanoparticles and viruses. Ozcan started a company called Holomic to commercialize the device, and his group has created smartphone apps for data analysis.
For more information, check out our Archives.
September 2017
Search this site with the power of