
Best Practices for Characterization of Magnetic Nanoparticles for Biomedical Applications
October 28, 2019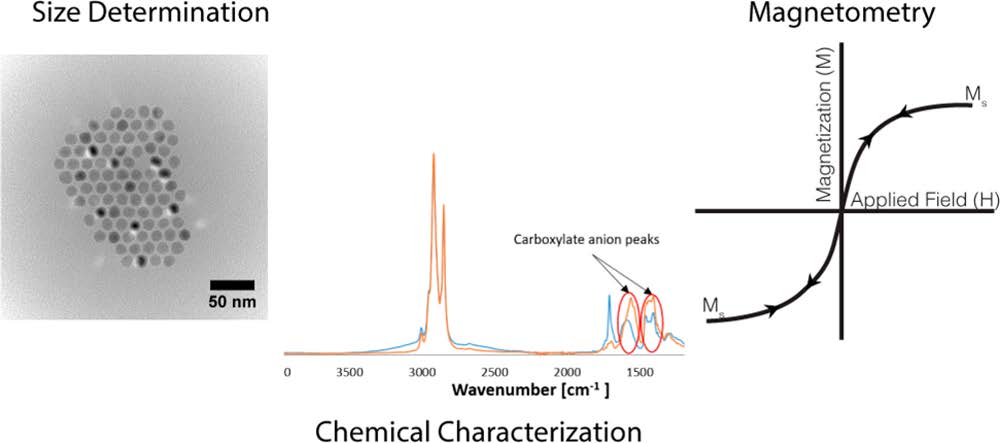 The use of magnetic nanoparticles in biomedical applications provides a wealth of opportunities. Nonetheless, to truly understand the interactions of these materials in biological media, detailed characterization is necessary with these complex systems. Prof. Thompson Mefford together with Sarah E. Sandler and Benjamin Fellows just published an article that might be very helpful to many of our colleagues.
The use of magnetic nanoparticles in biomedical applications provides a wealth of opportunities. Nonetheless, to truly understand the interactions of these materials in biological media, detailed characterization is necessary with these complex systems. Prof. Thompson Mefford together with Sarah E. Sandler and Benjamin Fellows just published an article that might be very helpful to many of our colleagues.
The article highlights some “best practices” in the analytical techniques and challenges in the measurement of the properties of these materials.
Check it out here, it is worth reading!
Complete JMMM Special Issue After the 2018 Meeting Is Now Available
October 07, 2019 It is our great pleasure to announce the official publication of the special JMMM issue after our 2018 meeting in Copenhagen.
It is our great pleasure to announce the official publication of the special JMMM issue after our 2018 meeting in Copenhagen.
At the end, we have now 75 interesting publications in our special issue. We hope you will all take the time to download, browse through the articles, and enjoy the new and interesting magnetic particle themes that all of your colleagues wrote about.
We make it easy for you - go to this website, and check them all out!
And then start planning for the next meeting in London, UK. Next June 2-5, 2020!
Watch Liquid-Based Magnet Droplets Twirl and Morph
July 23, 2019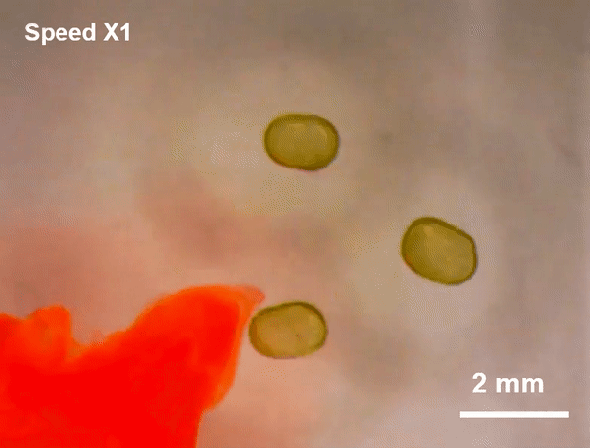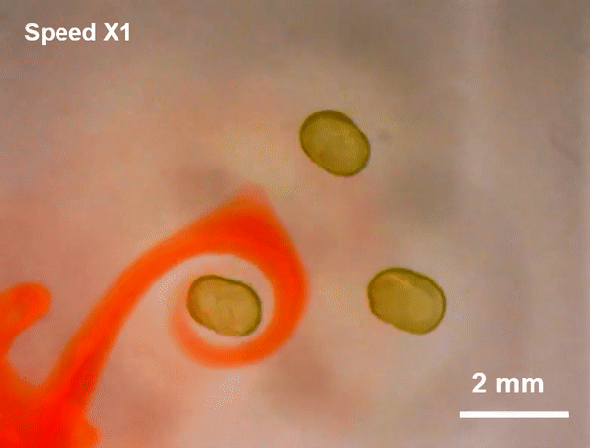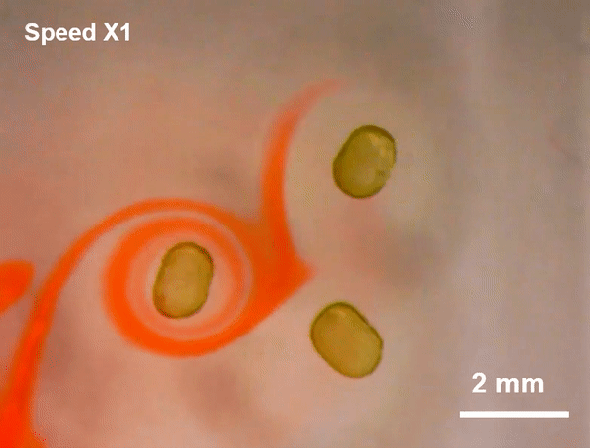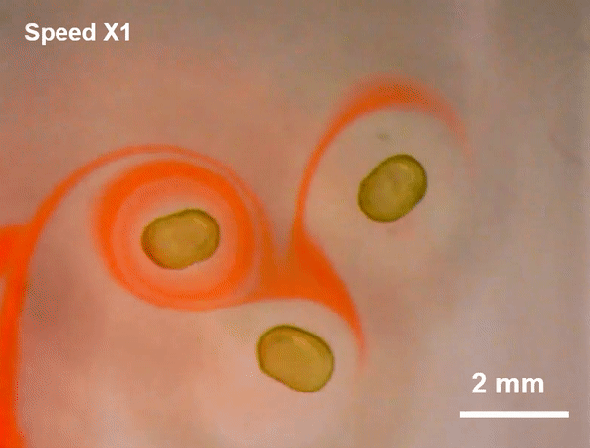 Researchers at Lawrence Berkeley National Laboratory (LBNL) have created a more malleable tool: a miniscule liquid-based magnet made from nanoparticles.
Researchers at Lawrence Berkeley National Laboratory (LBNL) have created a more malleable tool: a miniscule liquid-based magnet made from nanoparticles.
Such flexible magnets could be useful in places where rigid ones cannot go, including soft robots or flexible electronics. And although they are not yet ready for practical applications, the liquid-based magnets reveal a new facet of nanoparticle behavior, which could pave the way for a novel range of magnetic materials, the researchers say.
To create the liquid-based magnets, researchers led by Thomas P. Russell, a polymer scientist at the University of Massachusetts Amherst and a visiting researcher at LBNL, started with a modified 3-D printer. First, they printed millimeter-sized droplets of liquid filled with magnetic nanoparticles. This liquid-particle mix is superparamagnetic—it is strongly attracted to a magnetic field, but as soon as the field disappears, so does the magnetism. In plain language, it is magnetic but not a magnet—a characteristic typical of fluids that can be magnetized, called ferrofluids.
Bubble Magnetometry Highlights Nanoparticle Heterogeneity and Interaction
June 10, 2019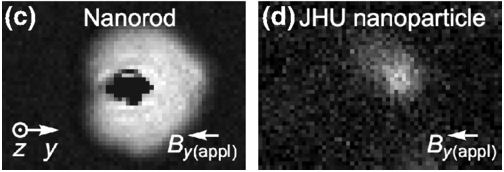
Check it out here.
Magnetic Nanoparticles Can Fix Broken Electric Wires
June 03, 2019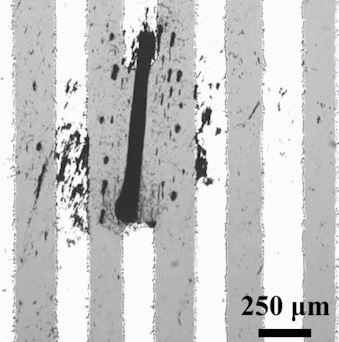 Inspired by armies of ants that link together to form living structures such as bridges and rafts, researchers have directed millions of nanoparticles to span the gap between two electrodes, forming an electrical wire (ACS Nano 2019, DOI: 10.1021/acsnano.9b02139). The technology could provide a new way to repair broken microcircuits and make tiny programmable microswitches, the researchers say.
Inspired by armies of ants that link together to form living structures such as bridges and rafts, researchers have directed millions of nanoparticles to span the gap between two electrodes, forming an electrical wire (ACS Nano 2019, DOI: 10.1021/acsnano.9b02139). The technology could provide a new way to repair broken microcircuits and make tiny programmable microswitches, the researchers say.
Roboticists dream of emulating the natural swarming behavior of insects and birds to make robots that work together on construction tasks or search-and-rescue missions. Scientists have also been engineering swarming particles that could have applications on the microscopic scale, such as tracking down tumors and delivering drugs.
Li Zhang, a mechanical and automation engineer at the Chinese University of Hong Kong, and his colleagues are designing microswarms for electronic applications. In 2018, they reported using oscillating magnetic fields to control millions of magnetic iron oxide nanoparticles in an ethanol suspension (Nat. Commun. 2018, DOI: 10.1038/s41467-018-05749-6). Changing the field’s strength relative to the fluid’s resistance made the particles line up into ribbon-like chains. By programming other parameters of the magnetic field, such as the oscillation frequency and angle, the researchers could elongate and shorten the ribbons, and make the nanoparticles split up and regroup. They could also make the swarms move through a maze.
Magnetic Bacteria Cause a Stir
May 19, 2019
Carrying out chemical reactions in Pickering emulsions avoids the large temperature and concentration gradients associated with traditional reaction systems. ‘But still, we need to find a way to stir the solution inside these micro-droplets. And this is the reason why we carried out this study to develop nano-sized stirrers,’ explains Weiguo Song from the Chinese Academy of Sciences.
For more information, check out https://www.chemistryworld.com/news/magnetic-bacteria-stir-reaction-in-droplet-microreactor/3008713.article.
Toyota Promises Cheaper Magnets for Electric Cars
May 19, 2019Japanese carmaker Toyota says it can cut the cost of rare earth element-containing magnets within the motors of electric vehicles by replacing up to half the neodymium in them with the more abundant, lower-cost rare earths lanthanum and cerium.
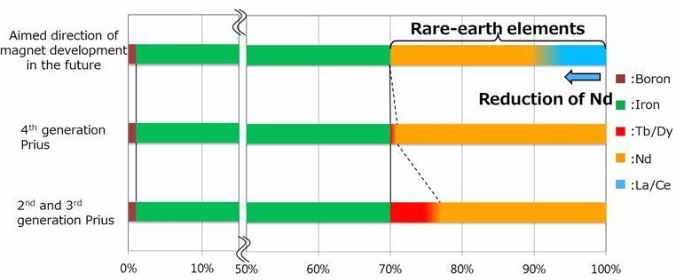
For more information, check here.
A Blood Test for Alzheimer's Disease Draws Near
March 28, 2019Anew approach with groundbreaking sensitivity is a major upgrade to a classic technique known as enzyme-linked immunosorbent assay (ELISA). The Massachusetts-based firm Quanterix has developed an automated version of ELISA that is 1,000 times as sensitive as older systems. Instead of tethering antibodies to the floor of wells in a microtiter plate, which usually holds multiple antibodies in each well, the company’s assay uses antibodies tethered to individual beads, which come to rest in individual wells on a grid. Each well is only 50 fL—about half a billionth the volume of a microtiter plate well—and holds only a single bead. By diluting plasma samples and adjusting the ratio of beads to protein molecules, researchers can ensure that each bead carries only one bound protein. An automated digital detection system measures the glow that results from a chemiluminescent reaction that occurs when the protein is present, allowing the assay to detect single protein molecules. These adjustments to dilution, reaction volumes, and standardization—coupled with antibodies that are highly specific to the peptide biomarkers—overcome problems including amyloid stickiness and ensure that newer assays outperform older attempts.
The Quanterix assay is 1,000-fold as sensitive as traditional enzyme-linked immunosorbent assays because it uses antibody-carrying magnetic beads that fit individually into femtoliter-volume wells. If a plasma protein of interest is present, the detection antibody linked to an enzyme binds to it. The enzyme goes on to cleave a chemiluminescent signaling molecule that can be detected in the well.
For more information, check out our Archives.
September 2017
Search this site with the power of
